Surrogate Endpoint
Surrogate Endpoint
A surrogate endpoint is a biomarker intended to substitute for a clinical endpoint.
What is a surrogate endpoint?
A surrogate endpoint can be a laboratory measurement, radiographic image, physical sign, or other measures that is not itself a measure of clinical benefit but is reasonably likely to predict clinical benefit. Surrogate endpoints are used instead of clinical endpoints, such as longer overall survival or improved quality of life.
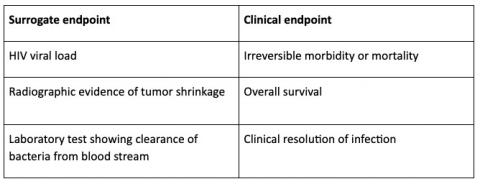
What are examples of surrogate endpoints?
A well-known example of a surrogate endpoint is blood pressure with the relevant clinical endpoint being a stroke. Other examples are (1):
Why are surrogate endpoints used?
The use of a surrogate endpoint in clinical trials can considerably shorten the time required to receive FDA approval and therefore make potentially life-saving drugs available to patients more quickly. Surrogate endpoints are also used in cases where the clinical benefit of improving the surrogate endpoint is well understood. Controlling blood pressure or blood sugar levels are examples of that.
How are surrogate endpoints validated?
Extensive testing and evidence gathering is necessary before a surrogate endpoint is accepted in place of a clinical outcome. Evidence includes, for example, epidemiological studies and clinical trials. Critically, clinical studies have to show that a surrogate endpoint is a reliable predictor of and correlates with the clinical benefit. Once a surrogate endpoint has undergone this validation process, the FDA refers to it as a validated surrogate endpoint and accepts it as evidence of benefit. (2)