eBook
Launching your medical device: A strategy and execution cross-functional guide
Launching new medical devices can be a lengthy and complex process. To be successful, you need clarity across every aspect of a product launch that is worth considering.
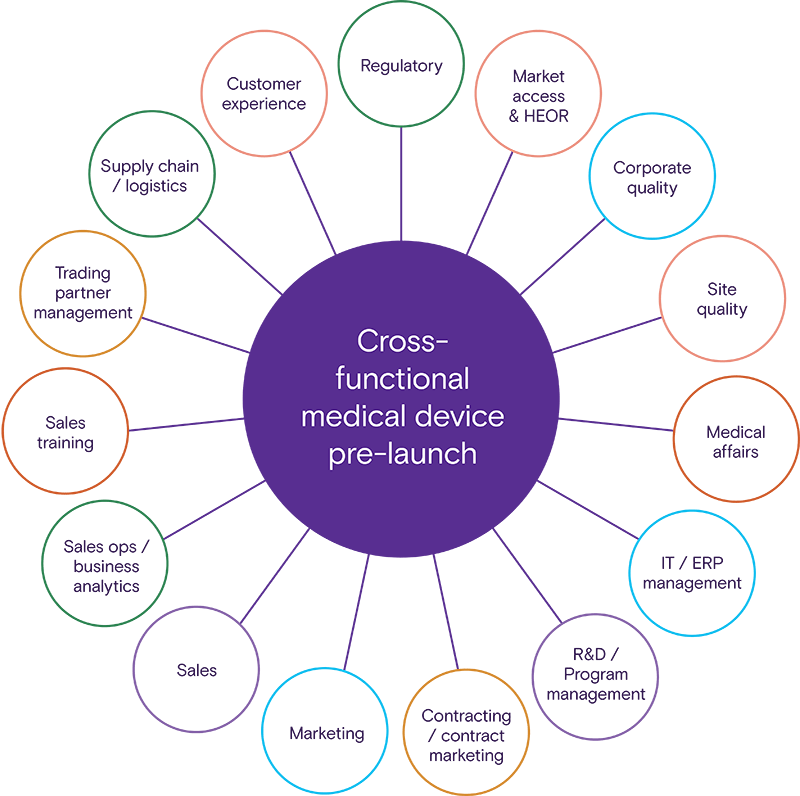
This comprehensive eBook captures the total cross-functional alignment needed to launch your device by outlining tactical and strategic considerations for 15 functional areas.
You have so much opportunity to succeed. Use this guide to develop a robust, solid launch strategy. You’ll get:
- Tactical checklists by function
- Pre-populated and editable GANTT chart to keep you organized
- Strategic considerations for your launch
Get your copy now!
What's inside
Access strategic guidance across key departments
We cover every step, team, and tactic to guide your device launch strategy and transform the healthcare landscape by improving patient outcomes, quality of life, or clinician workflow.
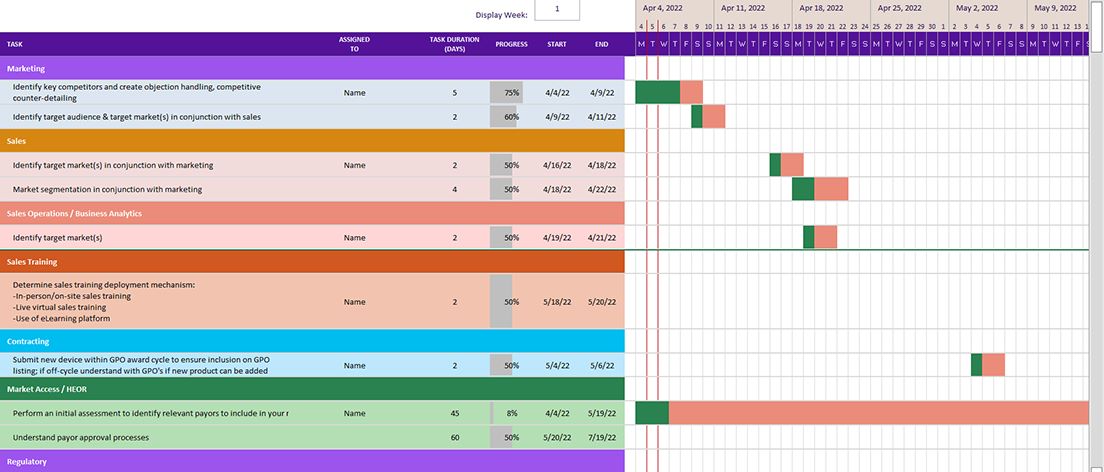
Keep all objectives of your launch strategy organized and actionable
Our included GANTT chart tool comes pre-populated with the tactical items in the eBook.