About a year ago, I received a letter in the mail from my car manufacturer saying they had initiated a recall on more than a million vehicles.
As it turned out, my car model was part of that group, affected by a glitch in the backup camera that would cause the live feed to disappear when the car shifted into reverse. I was told to venture over to my nearest dealership where they could perform the necessary software updates needed to fix the problem.
Being the focused, responsible person that I am, I left the notice on top of a stack of other papers where it was promptly forgotten about for three months. I could still use my car, I just had to silently acknowledge the technical problem I had yet to fix.
When I finally got around to the dealership to resolve this issue, the technician told me two things: first, I had missed my window to get the service done free of charge, and second, I was putting my safety at risk by using a faulty camera.
The moral of the story is to be aware of when a product you use has been recalled—and to stop using the product or get it fixed as soon as possible.
So, what’s the point?
Well, in recent months, the Food and Drug Administration has been winding down its issuance of emergency use authorizations (EUAs) on select medical devices, drugs, and tests. A recall, if you will, on certain personal protective equipment, diagnostic tests, antibody treatments and more.
This poses a significant financial and legal risk to healthcare providers using products and services no longer approved by the FDA. Providers and the manufacturers of products approved through emergency use authorizations need to be aware of changes in FDA designations and what to do if approval has been revoked or terminated.
What is an emergency use authorization?
An emergency use authorization is a designation given by the FDA that temporarily approves the use of certain medical products.
When a public health emergency like COVID-19 strikes, the FDA can issue emergency use authorizations so potentially life-saving treatments and equipment are delivered to the people who need it most.
The Moderna, Johnson & Johnson, and Pfizer coronavirus vaccines were all administered in the United States under emergency use authorization. A quick aside: the Pfizer vaccine moved beyond its emergency use status and was granted full approval by the FDA on August 23, 2021.
There are two important elements of emergency use authorization that are important to consider:
- A product approved for use through an emergency use authorization has not gone through the traditional, lengthier full approval process. According to the FDA, these products are only approved when there are no adequate, approved or available alternatives. When the potential benefits of the device outweigh any potential risks. This means that these products could potentially threaten patient safety and health.
- A product’s approval through an emergency use authorization is temporary, lasting only as long as the health emergency, or until the FDA revokes the authorization. Once this happens, any protections afforded vanish, leaving providers vulnerable to legal challenges if they choose to continue using unapproved devices. This can be especially dangerous for healthcare providers caught unaware—as emergency use authorizations can be revoked at any time.
With certain emergency use authorizations being revoked, healthcare providers using these unapproved products may find themselves facing a difficult challenge.
On the one hand, using these products could potentially endanger the safety of a patient, which could leave the provider liable and at risk of a lawsuit.
And on the other hand, healthcare providers need to have procedures in place to ensure that, when they transition away from EUA products, it doesn’t impact their ability to provide care and other services.
What can healthcare providers do?
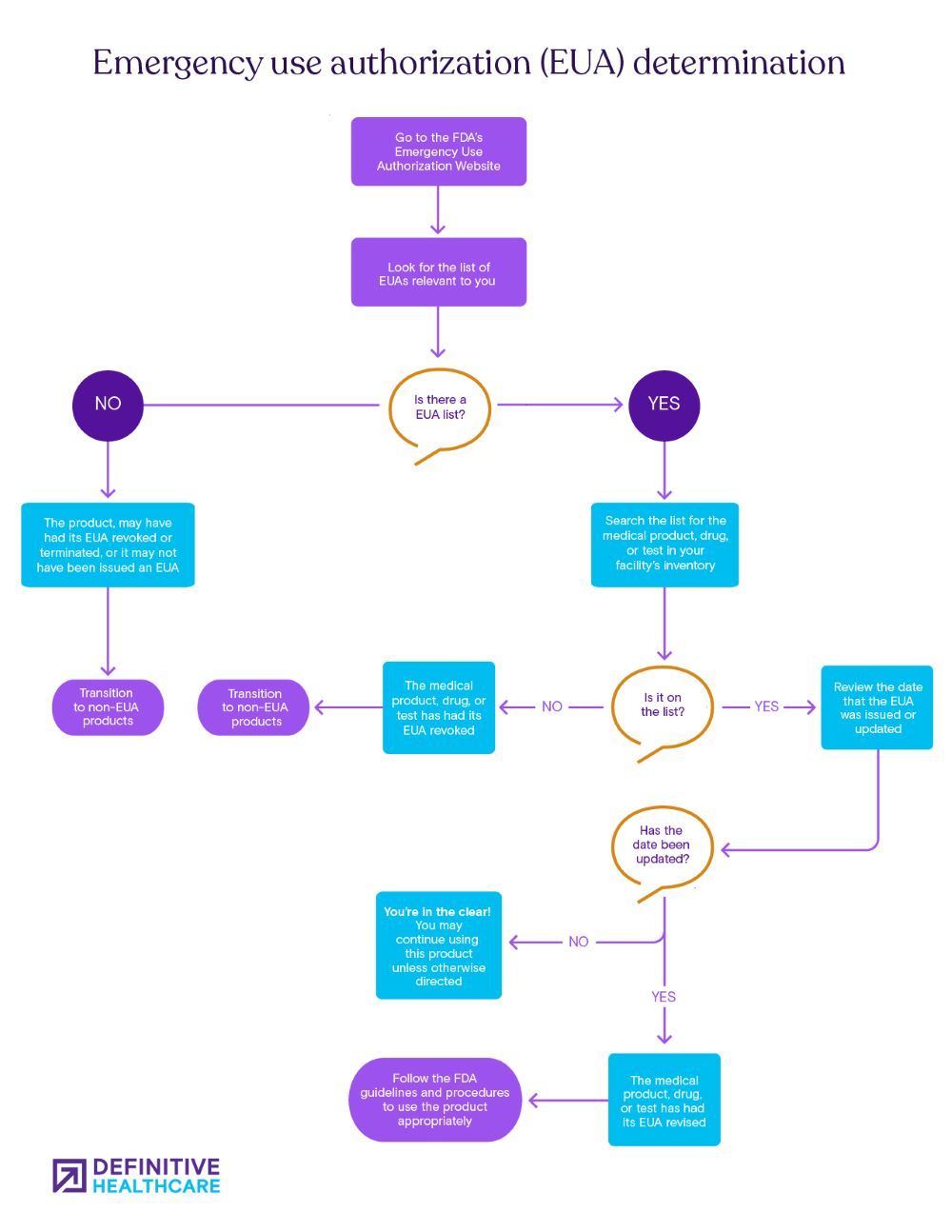
If you’re a healthcare provider, there are a few steps you can take to stay prepared if a product you’re using has been impacted by a change in EUA status.
Below, we’ve put together a useful decision tree diagram to help you map out the choices your organization needs to make.
While these decisions are being considered, it’s important to begin tracking and inventorying the drugs, personal protective equipment, devices and other products your organization use that are approved under an emergency use authorization.
It’s also critical to be aware of when the FDA makes a change in an emergency use authorization. The FDA keeps a constantly updated list of ongoing EUAs here. You can also find a list of revoked EUAs archived here.
If a EUA has been revoked or terminated, any affected products should be fully documented and stored away. If any changes in a EUA’s status have occurred, then be sure to communicate them to your organization’s staff to ensure that these products aren’t used. Communication with your supplier is also important as a revoked EUA on a product may affect ongoing or future contracts.
The prevailing future of emergency use authorizations is uncertain. While the FDA has taken steps to revoke or terminate EUAs in recent months, it’s difficult to predict how the spread of the Delta variant may change the government’s response.
For biopharmaceutical and medical device companies, the shifting healthcare landscape could impact how they do business with healthcare providers, how they market their products and the procedures they must follow to secure full approval with the FDA.
Definitive Healthcare can provide healthcare commercial intelligence that helps these companies develop and accelerate their go-to-market strategy and effectively adapt to changes in the market. Learn about more solutions for biopharma and medical device companies today.